
Computational Chemistry and Physics on the Nanoscale
CCPN 2009

Navigation
Chemical Speciation in aqueous solution using density-functional based methods
Hélio Anderson Duarte
Chemical Speciation plays an important role in many processes occurring in water medium. Acids, bases, metal ions and many other organic molecules form, in solution, several species that can interact with each other leading to much complex systems. These species are, in the last instance, responsible for the chemical behaviour of such compounds. However, experimental measurements of such molecules, in situ, are rather difficult since many species are in equilibrium, which can be displaced very easily by modifying the thermodynamic conditions.
An extension of the chemical speciation is the adsorption on the liquid/solid interface. In many environmental and technological processes, the water plays crucial role modifying the solid surface and enhancing its adsorption properties. The accurate description of such systems has to include adequately the solvent effects in the modelling.
The topics of the course will cover an introduction to the chemical speciation, stability constants of the complexes and the experiments to estimate them. Different approaches to include the solvent effects in the modelling will be discussed. The modelling of the liquid/solid interface will be discussed and examples will be given. In the tutorials, students will have opportunity to work with real problems related to chemical speciation and propose strategies to approach them.
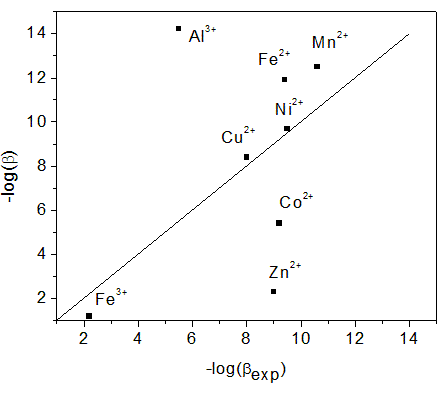
Estimated versus experimental values of –log(beta) for the first hydrolysis reaction of transition metal ions in solution
 General Information
General Information