Layer-layer separation
The H2 penetration between the two graphene layers may be hypothetically presented as a two stage process.
First the layers should be separated so that molecular hydrogen can diffuse (second stage) in between.
Since the separation process is highly endothermic it would be thermodynamically impossible for H2 to penetrate between the
layers of graphene even though that the physisorption itself will partially compensate this effect.
According to the dispersion corrected [73,74] (DC) density functional based tight binding (DFTB) method[75] as implemented
in the deMon [76] code, it requires 74 meV per carbon to increase the interlayer distance of graphite to 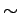 6 Å.
That is, to the optimal distance where H2 could penetrate into the material.
If all low energy positions of the material are statically covered with H2, six carbon atoms bind one hydrogen molecule(see Fig. 2.6), introduction of H2 will stabilise the opened graphite layer, but only by about 125 meV per H2.
In total, the penetration of H2 into graphite is an endothermic process of at least 320 meV per H2.
Therefore, the interlayer distance of graphite needs to be increased by other means, and, indeed, several possibilities have been discussed in the literature.
They include functionalisation of the sheets, for example by fluorination [77,78], by doping with Li complexes [22] or by oxidation with aquas acid agents, [79,80] forming randomly spread out hydroxy and epoxide groups which increase the interlayer distance.
Functionalised graphite can include interlayer distances from 4.7 to 9 Å, depending on preparation and temperature.
As functionalisation transforms
6 Å.
That is, to the optimal distance where H2 could penetrate into the material.
If all low energy positions of the material are statically covered with H2, six carbon atoms bind one hydrogen molecule(see Fig. 2.6), introduction of H2 will stabilise the opened graphite layer, but only by about 125 meV per H2.
In total, the penetration of H2 into graphite is an endothermic process of at least 320 meV per H2.
Therefore, the interlayer distance of graphite needs to be increased by other means, and, indeed, several possibilities have been discussed in the literature.
They include functionalisation of the sheets, for example by fluorination [77,78], by doping with Li complexes [22] or by oxidation with aquas acid agents, [79,80] forming randomly spread out hydroxy and epoxide groups which increase the interlayer distance.
Functionalised graphite can include interlayer distances from 4.7 to 9 Å, depending on preparation and temperature.
As functionalisation transforms 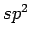 carbon to
carbon to 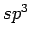 , it allows covalent interlayer bonds and suggest weakening of the interaction potential by reducing the carbon sp
, it allows covalent interlayer bonds and suggest weakening of the interaction potential by reducing the carbon sp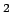 contributions[81].
However, if the transformations from
contributions[81].
However, if the transformations from 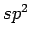 to
to 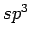 do not appear too often the functionalisation of graphite may lead to opposite effects as discussed later (see Chapter 6).
An alternative possibility to increase the interlayer distance with leaving the graphene sheets intact is the intercalation of spacer molecules [82].
Thermodynamically, during the intercalation process, a destabilising process (the interlayer van der Waals bonds are broken) is partially compensated by the formation of spacer layer interactions.
The intercalation of spacers into graphite is, however, possible: recently, the synthesis and characterisation of graphite with intercalated C60 fullerenes has been reported. [82]
The fullerene cages form a hexagonal two-dimensional lattice between the layers, and the transmission electron microscopy (TEM) image indicates that no covalent bonds between the fullerenes nor between them and the layers, are present.
The spacer
do not appear too often the functionalisation of graphite may lead to opposite effects as discussed later (see Chapter 6).
An alternative possibility to increase the interlayer distance with leaving the graphene sheets intact is the intercalation of spacer molecules [82].
Thermodynamically, during the intercalation process, a destabilising process (the interlayer van der Waals bonds are broken) is partially compensated by the formation of spacer layer interactions.
The intercalation of spacers into graphite is, however, possible: recently, the synthesis and characterisation of graphite with intercalated C60 fullerenes has been reported. [82]
The fullerene cages form a hexagonal two-dimensional lattice between the layers, and the transmission electron microscopy (TEM) image indicates that no covalent bonds between the fullerenes nor between them and the layers, are present.
The spacer graphene and space
graphene and space spacer distances for the most compact configurations are expected to be nearly the same (between 3 and 3.5 Å).
However, the distance between fullerenes can vary as they are not covalently bonded and their density can change easily.
spacer distances for the most compact configurations are expected to be nearly the same (between 3 and 3.5 Å).
However, the distance between fullerenes can vary as they are not covalently bonded and their density can change easily.
The fully DC DFTB optimised geometry [83] of C60
DFTB optimised geometry [83] of C60 intercalated graphite agrees well with the reported TEM image of Gupta and
co-workers.
Moreover, the C60-intercalated fullerene material (shortly CIG) has an atomisation energy per atom of
intercalated graphite agrees well with the reported TEM image of Gupta and
co-workers.
Moreover, the C60-intercalated fullerene material (shortly CIG) has an atomisation energy per atom of  8.709 eV/atom.
A value which is 170 meV/atom lower than that of free or solid C60, but about 340 meV/atom higher than for graphite (Table 4.1) indicating for a relatively stable structure.
8.709 eV/atom.
A value which is 170 meV/atom lower than that of free or solid C60, but about 340 meV/atom higher than for graphite (Table 4.1) indicating for a relatively stable structure.
Lyuben Zhechkov
2007-09-04
![]() DFTB optimised geometry [83] of C60
DFTB optimised geometry [83] of C60![]() intercalated graphite agrees well with the reported TEM image of Gupta and
co-workers.
Moreover, the C60-intercalated fullerene material (shortly CIG) has an atomisation energy per atom of
intercalated graphite agrees well with the reported TEM image of Gupta and
co-workers.
Moreover, the C60-intercalated fullerene material (shortly CIG) has an atomisation energy per atom of ![]() 8.709 eV/atom.
A value which is 170 meV/atom lower than that of free or solid C60, but about 340 meV/atom higher than for graphite (Table 4.1) indicating for a relatively stable structure.
8.709 eV/atom.
A value which is 170 meV/atom lower than that of free or solid C60, but about 340 meV/atom higher than for graphite (Table 4.1) indicating for a relatively stable structure.