Dislocations in graphite
Graphite is widly used as moderator in nuclear reactors. Under irradiation its properties undergo significant changes because of the produced defects.
The primary defects in this type of modified graphite (MG) are usually interstices and vacancies, but they can aggregate as well by forming extended defects such as lines or dislocation loops[91].
By applying the tools of quantum chemistry Suarez-Martinez et. al. [92] have recently reported stable C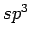 atoms in the dislocation core.
Two dislocation types have been characterised: a zigzag prismatic edge dislocation which implies the presence of C
atoms in the dislocation core.
Two dislocation types have been characterised: a zigzag prismatic edge dislocation which implies the presence of C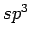 atoms and an armchair prismatic edge dislocation (no C
atoms and an armchair prismatic edge dislocation (no C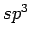 atoms).
These varieties of graphite are found to be as stable as the non modified graphite.
Their structures together with the H2
atoms).
These varieties of graphite are found to be as stable as the non modified graphite.
Their structures together with the H2 host potential interaction can be seen in Figures 6.1 and 6.2,
host potential interaction can be seen in Figures 6.1 and 6.2,
Figure:
H2 interaction potential in zigzag dislocated graphite. The
colour of the iso-surfaces corespond to the energy bar in kJ
interaction potential in zigzag dislocated graphite. The
colour of the iso-surfaces corespond to the energy bar in kJ mol
mol .
.
|
Figure:
H2 interaction potential in armchair dislocated graphite. The
colours of the iso-surfaces corespond to the energy bar in kJ
interaction potential in armchair dislocated graphite. The
colours of the iso-surfaces corespond to the energy bar in kJ mol
mol
|
which show that well deffined cavities appear due to the bent graphene surfaces.
The cavities for both modifications have a width of  7 Å, which is about the same as the optimal distance (for H2 storage) found for a slit pore in the hypothetical graphite (discussed in Chapter 3).
The dislocations in this case take the role of spacers, similarly to the fulerenes in the CIG structure (discussed in Chapter 4), but being a natural part of the graphene layers (e.g. they can not move freely as in the CIG case).
This demonstrates a possibility to create porous modifications of graphite by taking advantage of naturally or artificially created defects.
Such periodic modifications experience maximum H2 physisorption interaction free energy of about 10 kJ
7 Å, which is about the same as the optimal distance (for H2 storage) found for a slit pore in the hypothetical graphite (discussed in Chapter 3).
The dislocations in this case take the role of spacers, similarly to the fulerenes in the CIG structure (discussed in Chapter 4), but being a natural part of the graphene layers (e.g. they can not move freely as in the CIG case).
This demonstrates a possibility to create porous modifications of graphite by taking advantage of naturally or artificially created defects.
Such periodic modifications experience maximum H2 physisorption interaction free energy of about 10 kJ mol
mol (at 300 K).
According to the symulation, the estimated volumetric capacity (Fig. 6.3) easily reach the target value for 2010 (V, 45 cm
(at 300 K).
According to the symulation, the estimated volumetric capacity (Fig. 6.3) easily reach the target value for 2010 (V, 45 cm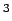 /mol)[4] at moderate pressure of about 5 MPa (see Fig. 6.3).
/mol)[4] at moderate pressure of about 5 MPa (see Fig. 6.3).
Figure:
Volumetric (Top) and gravimetric (Bottom) storage capacities for solid different types
of dislocated graphite, given for various temperatures (see the legend) and pressures.
The symbols on the curves denote that the approximation is within the limits (pressure and temperature) of the real gas
equation of state [36]. The targets[4] for automotive
applications (Gwt) 6.0 %,(V) 44.4 cm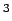 /mol) are indicated as horizontal lines.
/mol) are indicated as horizontal lines.
|
To fulfil the gravimetric targets (4.5 and 6 wgt % for respectively 2007 and 2010 ) requires some more rigorous thermodynamical conditions (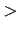 15 MPa at 300 K see Fig. 6.3), due to the relatively high carbon density6.1 of these graphite modifications
However, gravimetric H2/carbon ratio of about 4 % at 5 MPa and 300 K already exceeds the best known capacities for physisirption substrates.
15 MPa at 300 K see Fig. 6.3), due to the relatively high carbon density6.1 of these graphite modifications
However, gravimetric H2/carbon ratio of about 4 % at 5 MPa and 300 K already exceeds the best known capacities for physisirption substrates.
Lyuben Zhechkov
2007-09-04